Research Projects
We’re interested in using technology to solve problems facing physicians and scientists as they attempt to diagnose, treat, and understand disease.
Our Tools
Most members of our lab use one or more of the technological platforms described below to investigate their application of interest.
Microfluidics
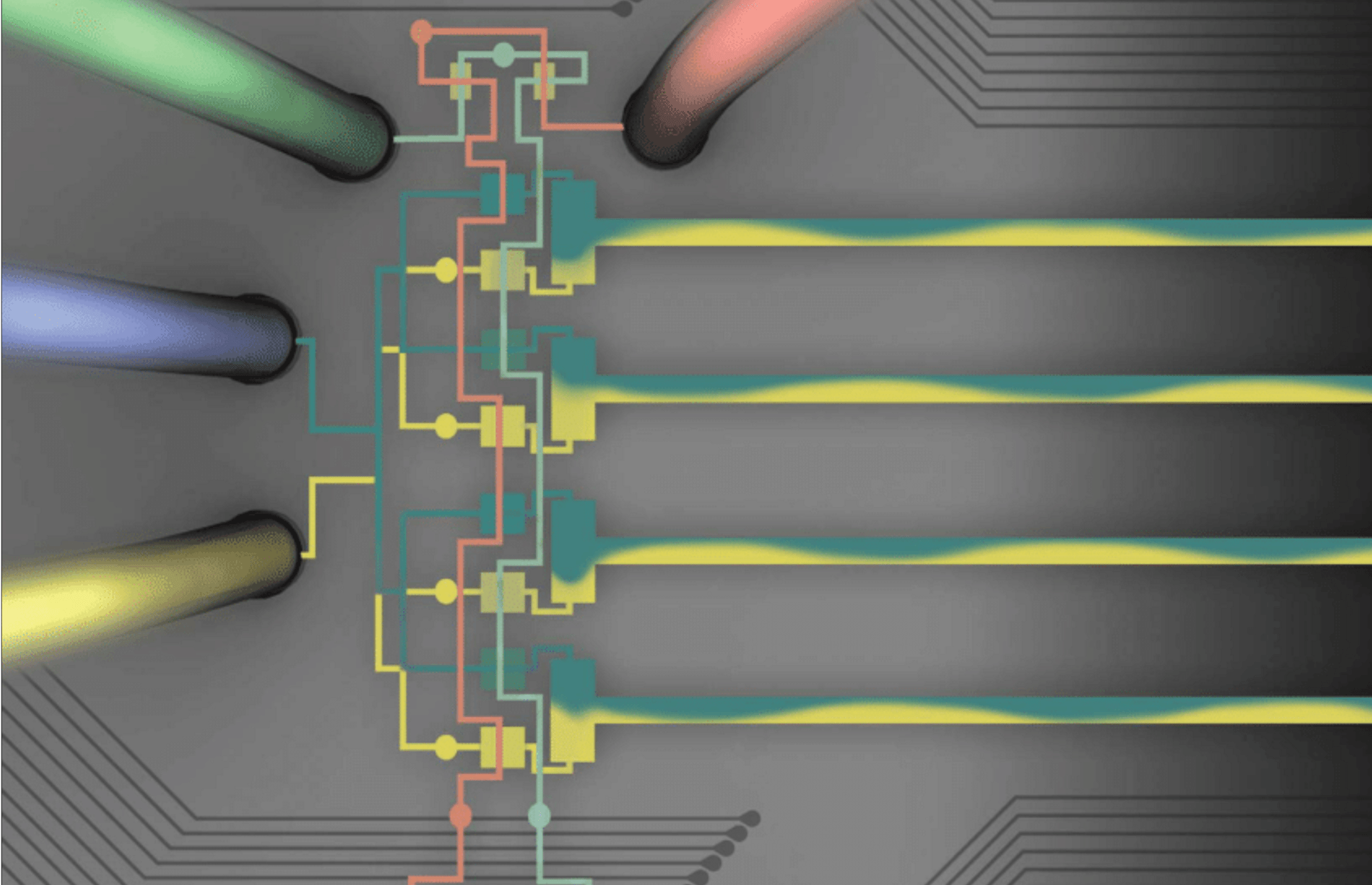
 Broadly, microfluidics are any systems having compartments or conduits at the nano- or micro-scale. Most of our microfluidics are constructed from a transparent, flexible rubber called PDMS, because it is low-cost, has high feature retention, and is compatible with cell culture. We use microfluidics to manipulate DNA, cells, and protein at the microscale; to generate tissue-mimetic microenvironments; and to design fluidic logic circuits that can be used in soft robots.
Broadly, microfluidics are any systems having compartments or conduits at the nano- or micro-scale. Most of our microfluidics are constructed from a transparent, flexible rubber called PDMS, because it is low-cost, has high feature retention, and is compatible with cell culture. We use microfluidics to manipulate DNA, cells, and protein at the microscale; to generate tissue-mimetic microenvironments; and to design fluidic logic circuits that can be used in soft robots.
We fabricate our microfluidics using photolithography for fine features, and we use our lab-owned micromill for rapid-prototyping and generating 3D structures.
Hanging Drop/3D Cell Culture
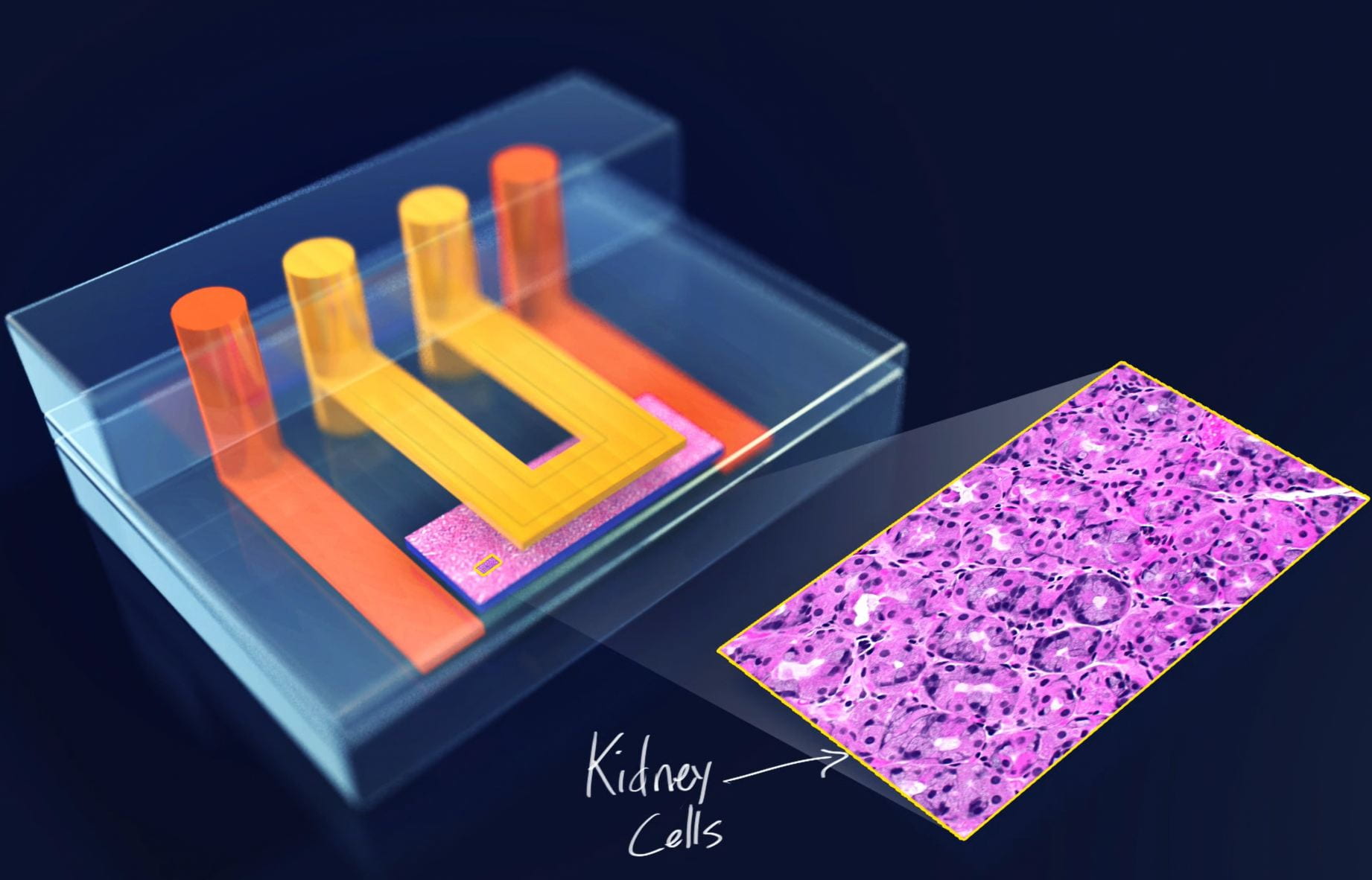
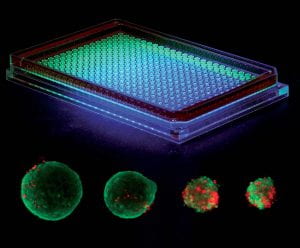 ShuLab uses our patented hanging drop array to generate high throughput spheroids and organoids to study a variety of conditions. A small drop of liquid (~25 uL) is suspended in each well, and cells are added to the droplet. Without anything to adhere to, most cell types, especially epithelial cells, will spontaneously form either a compacted or hollow sphere.
ShuLab uses our patented hanging drop array to generate high throughput spheroids and organoids to study a variety of conditions. A small drop of liquid (~25 uL) is suspended in each well, and cells are added to the droplet. Without anything to adhere to, most cell types, especially epithelial cells, will spontaneously form either a compacted or hollow sphere.
We are currently applying hanging drop culture to generate microtissues mimicking triple negative breast cancer. Recently, we have used hanging drop culture to invert the epithelial lumen to face the outside for facile analysis of exposures, extravasation, and drug efficacy.
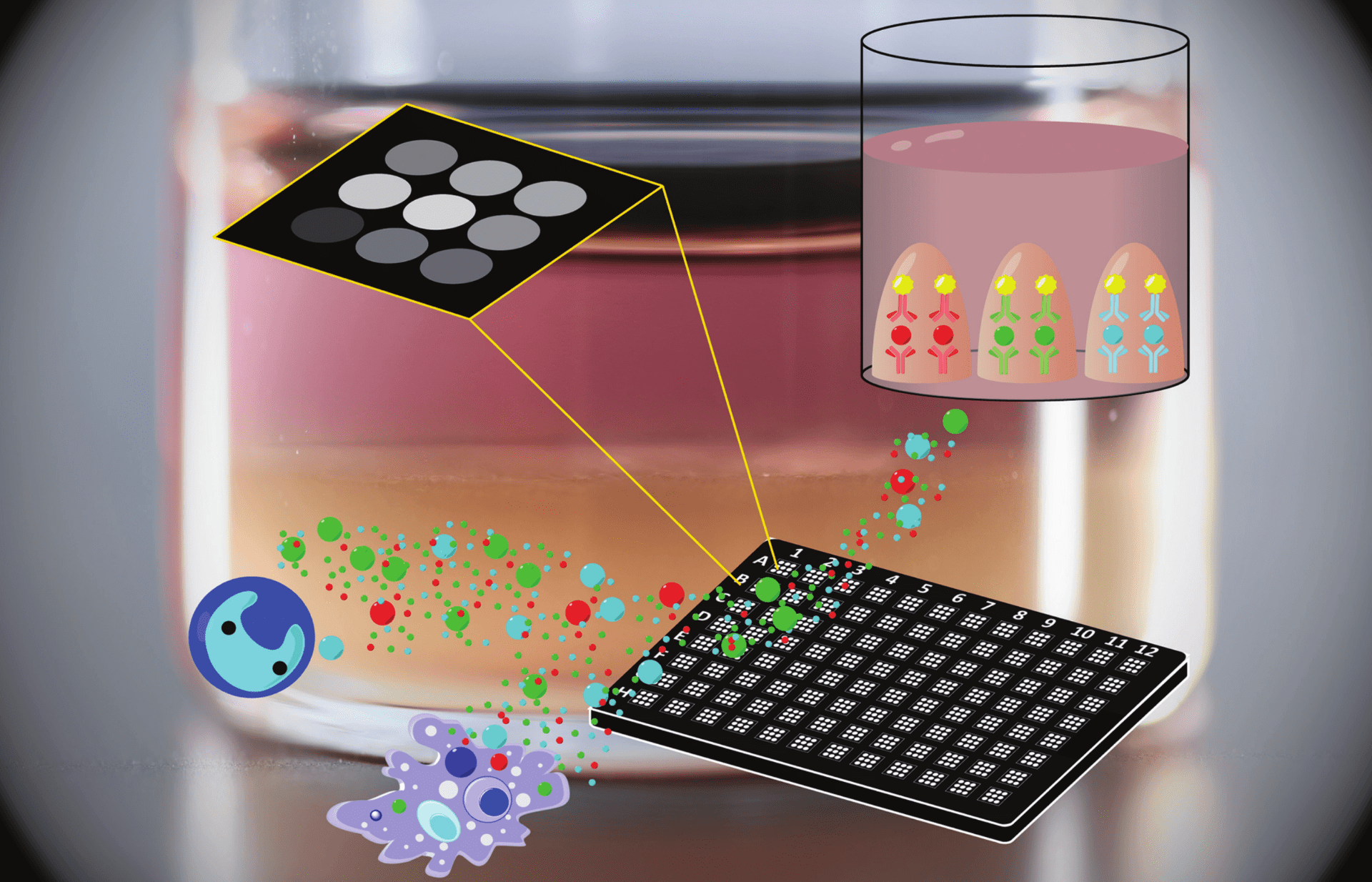
Aqueous Two-phase systems
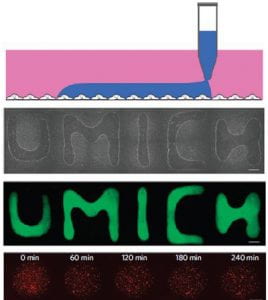 ATPS are formed when aqueous polymers with different hydrophobicity and intermolecular forces are mixed at a critical concentration. The polymers spontaneously partition into two phases with different composition. Our lab’s workhorse is the PEG-DEX ATPS, composed of poly(ethylene glycol) and dextran of varying molecular weights.
ATPS are formed when aqueous polymers with different hydrophobicity and intermolecular forces are mixed at a critical concentration. The polymers spontaneously partition into two phases with different composition. Our lab’s workhorse is the PEG-DEX ATPS, composed of poly(ethylene glycol) and dextran of varying molecular weights.
ShuLab exploits ATPS to perform cell- and bio-based separations that require aqueous media (e.g. we developed an ATPS-based method to separate extracellular vesicles from solution). We take advantage of the DEX phase’s high density to use ATPS for bioprinting of cells, viruses, bacteria, or proteins. We use ATPS to spatially control the location of proteins that preferentially partition into a single phase, such as antibodies in our multiplexed ATPS ELISA. We are also interested in naturally occurring ATPS at the micro-scale in cell chromatin and cytoplasm.
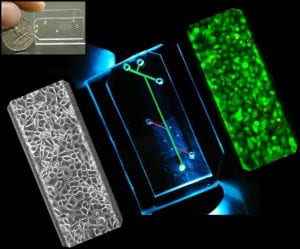
DNA-based Biomaterials
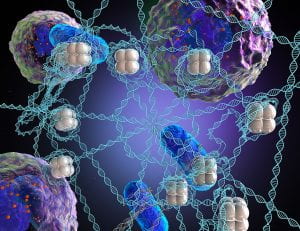 Neutrophils can expel their chromatin in tangled up webs called neutrophil extracellular traps (NETs). NETs are decorated with cytokines, proteases, and histones that influence the cellular microenvironment. Recently, we invented a biomimetic DNA material mimicking neutrophil extracellular traps (NETs) so that we can control the composition for a unique bottom-up approach to studying how NETs and their components function in health and disease.
Neutrophils can expel their chromatin in tangled up webs called neutrophil extracellular traps (NETs). NETs are decorated with cytokines, proteases, and histones that influence the cellular microenvironment. Recently, we invented a biomimetic DNA material mimicking neutrophil extracellular traps (NETs) so that we can control the composition for a unique bottom-up approach to studying how NETs and their components function in health and disease.
Our DNA microwebs are constructed from lambda phage- or salmon-derived DNA sonicated with calf-derived histone to create a bacteria-trapping, dendritic cell-stimulating NET-mimetic material. We can manipulate our microwebs by controlling their surface charge, decorating them with proteins or pathogens, and printing them in micro-scale droplets for controlled cell interaction.
Our Applications
Microphysiological Systems
Combining physical forces, engineered materials, and multiple cell types to recreate the tissue microenvironment from the bottom up.
Bioassays
Invention and improvement of protein- and cell-based assays that quantify cellular responses to drugs and organotypic stimuli.
Bioprocessing and micro/nanofabrication
Separations and spatial manipulation of biological material such as cells, proteins, nucleic acids, and bacteria.