BIOASSAYS
High throughput and small-scale are essential in many applications to keep costs low and efficiency & speed high. We’ve invented, miniaturized, and scaled up a variety of assays to improve preclinical studies and accelerate translation to clinical work.
Currently, we’re focusing on the following areas:
One-hour multiplex Enzyme-Linked ImmunoSorbent Assay (ELISA)
In a variety of lung diseases, the small airways and alveoli become flooded with liquid that interrupts oxygenation and causes the small, delicate airways and alveoli to collapse. In the airways
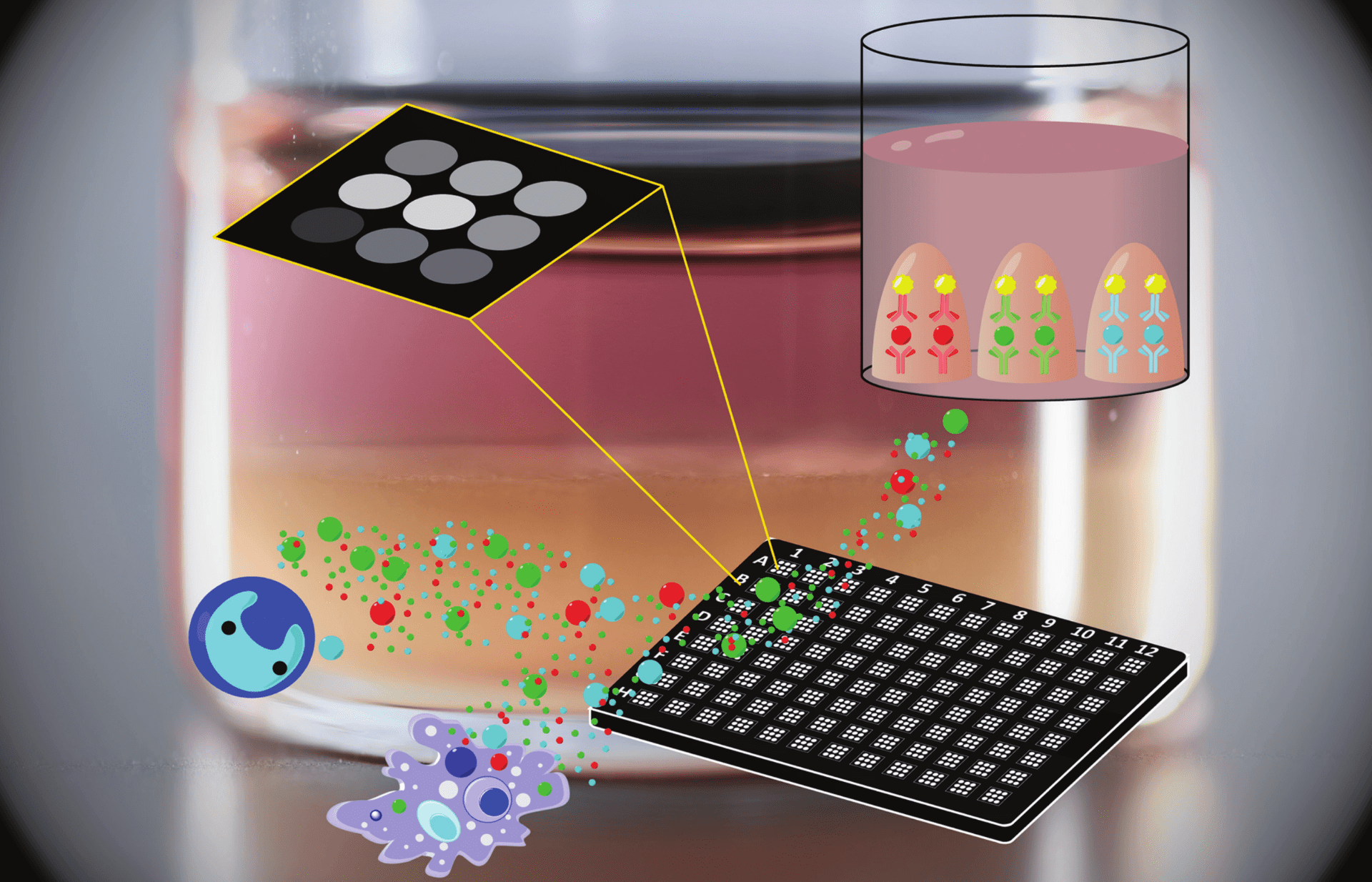
ELISA assays are a staple of laboratory and clinical investigation, and single-step and multiplexed platforms are emerging that can measure multiple targets at once without large sample volumes. In typical multiplex ELISAs, however, undesirable cross-reactivity between secondary antibodies of different targets limits their specificity and increases signal-to-noise.
We have developed an ATPS-based ELISA protocol requiring only one sample well to detect five targets with a single one-hour incubation step. This method increases the limit of detection up to 10x, and increases signal-to-noise ratio up to 3x, over a standard sandwich ELISA.
Current Lab members: Cameron Yamanishi, Midori Maeda
References: Tongdee 2020; Eiden 2016; Dixon 2015; Yamanishi 2015; Simon 2014; Frampton 2014
Assessment of triple negative breast cancer metastasis in high throughput organoid platform
Ductal carcinoma in situ (DCIS) is a non-invasive breast cancer that accounts for ≈25% of all breast carcinoma diagnoses. About 30% of untreated cases of DCIS develop invasive ductal carcinoma (IDC) within 10 years. At present, there are no biomarkers that can predict which cases of DCIS will progress to invasion. Therefore, this is a need for understanding of underlying factors responsible for the disease progression and for the development of prognostic biomarkers.
Our lab addressed this need by developing a high throughput ductal carcinoma invasion model that uses our patented hanging drop platform to create hollow, polarized organoids from MCF10A cells that model mammary duct epithelium. Our model is uniquely polarized with the basal surface on the interior, allowing convenient addition of invading cells on the luminal side as they would in vivo.
With our current platform, typically 192 organoids (every other well ofa 384-well plate) can be simultaneously assayed per plate and maintained in culture for 25+ days.
Current Lab members: Eric Parigoris, Soojung Lee, David Mertz
References: Parigoris 2020, Djomehri 2019
Small-volume, high throughput collagen contraction assay to evaluate fibrosis prevention drugs
Fibrosis is a progressive disease interfering with tissue structure and function, which stems from an aberrant wound healing response. Epithelial injury and clot formation lead to fibroblast invasion and activation, followed by contraction and remodeling of the extracellular matrix. Phenotypic assays, those that recapitulate a specific aspect of the fibrotic process, have proven generally useful in the discovery of first-in-class therapeutics and disease mechanisms.
Collagen gel contraction is the gold standard of phenotypic readouts for evaluation of fibrosis. A fibroblast-laden collagen gel is deposited in conditioned cell culture media to mimic the processes of fibroblast activation, collagen deposition, and contraction. However, these assays are typically performed manually in 6-well plates, limiting their translation for high throughput drug screening.
Our lab has adapted the traditional collagen contraction assay to a 384-well platform that uses liquid handling equipment for a fully automated, ,high throughput evaluation of fibrosis therapeutics for preclinical screening.
Current Lab members: Jon Chang
References: Yamanishi 2019, Leung 2015
Microphysiological Systems
Combining physical forces, engineered materials, and multiple cell types to recreate the tissue microenvironment from the bottom up.
Bioassays
Invention and improvement of protein- and cell-based assays that quantify cellular responses to drugs and organotypic stimuli.
Bioprocessing and micro/nanofabrication
Separations and spatial manipulation of biological material such as cells, proteins, nucleic acids, and bacteria.