MICROPHYSIOLOGICAL SYSTEMS
Microphysiological systems incorporate mechanical force, cell coculture, chemical or electrical gradients, and other factors to generate a complex microenvironment that mimics organ-level functions in vitro.
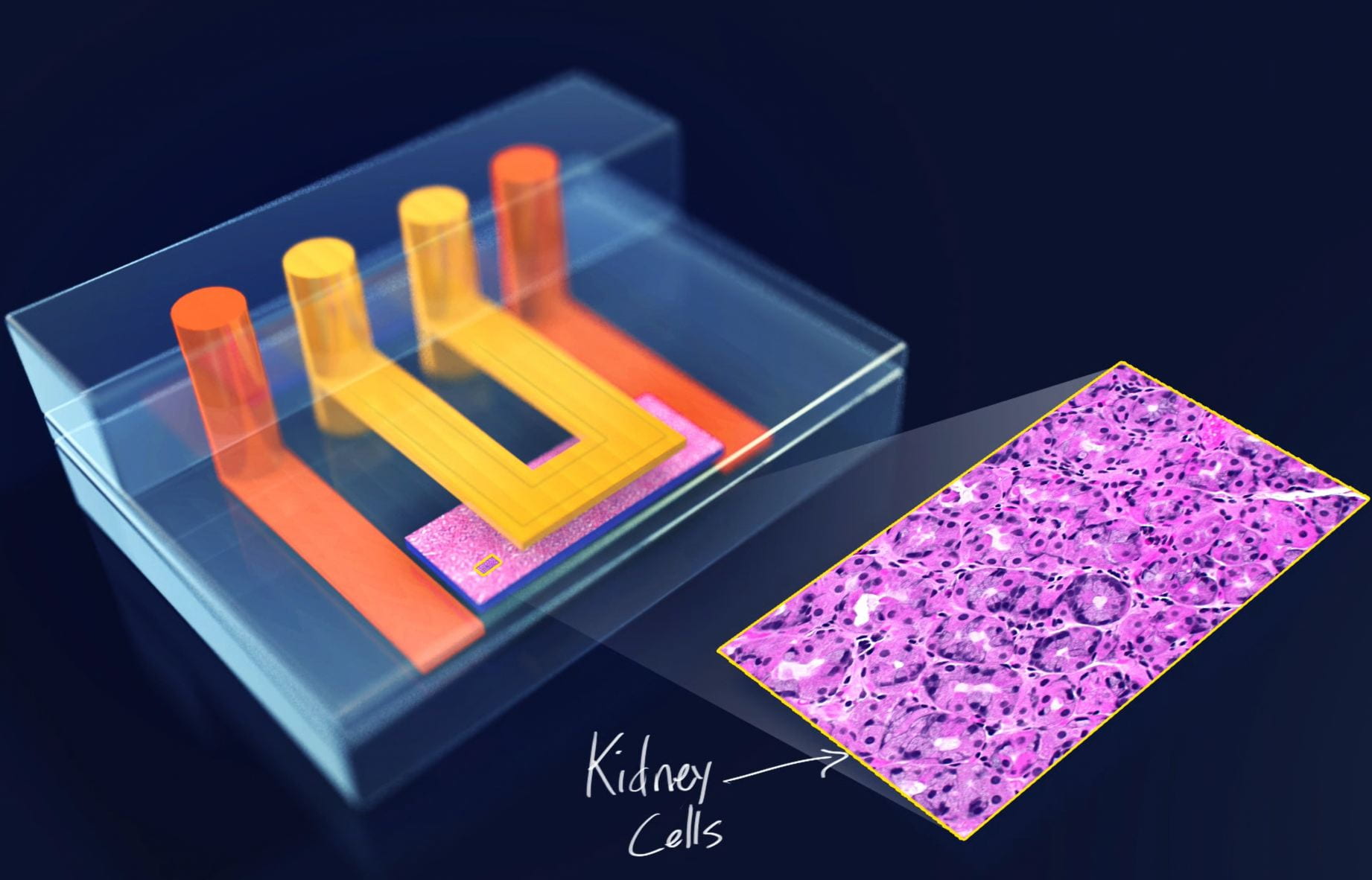
Over the years, we’ve used microfluidics, hanging drop culture, ATPS, and biomaterials to engineer a variety of cell systems that mimic organ-level function such as our nephrotoxicity-mimicking kidney-on-a-chip (Kim 2016, Biofabrication), our cancer extravasation platform ( Song 2009, Lab Chip) and our embryo culture system (Heo 2012, Lab Chip).
Currently, we’re focusing on the following areas:
Impact of fluid mechanical stress in the distal airways
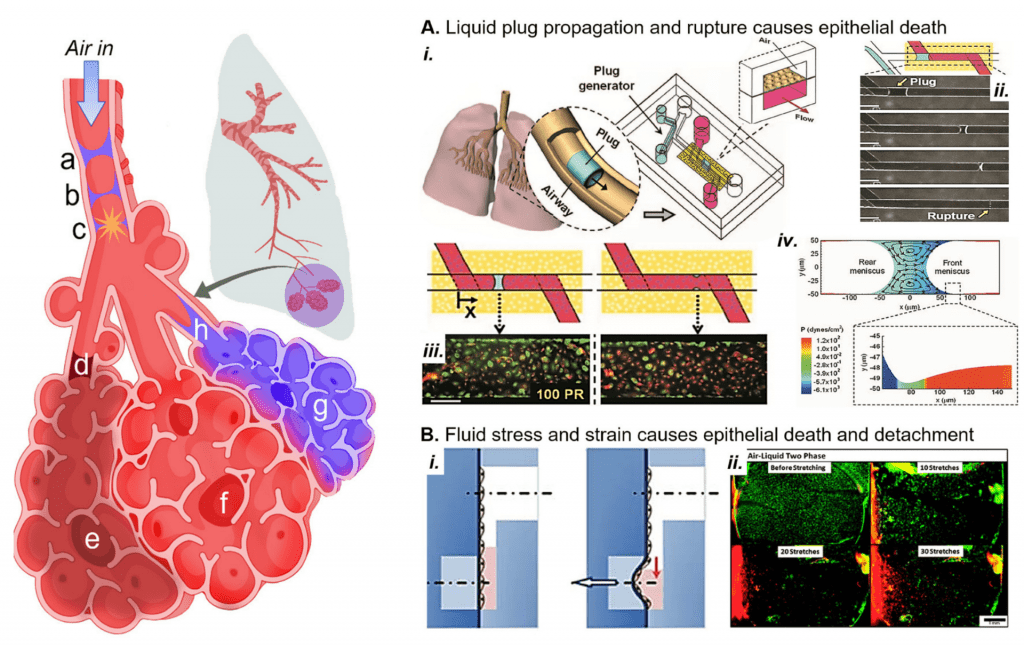
In a variety of lung diseases, the small airways and alveoli become flooded with liquid that interrupts oxygenation and causes the small, delicate airways and alveoli to collapse. In the airwaysOur lab has recapitulated the liquid plug propagation and rupture in a microfluidic lung-on-a-chip, where we showed that liquid plug propagation and rupture induces cell death under physiologic conditions. We are extending this model to study how sublethal fluid stress could induce inflammatory responses that contribute to the dysregulated immune environment present during acute respiratory distress syndrome, a common cause of pulmonary edema that leads to these liquid plugs forming in great numbers.
Lab members: Hannah Viola
Collaborators: James Grotberg, MD, PhD, Univ. of Michigan
References: Huh 2007; Tavana 2010; Tavana 2011; Viola 2019
Neutrophil extracellular traps in health and disease
Neutrophils are the most abundant white blood cell in the circulation, and the first to respond when there is an inflammatory insult such as tissue injury or infection. Therefore, they play a crucial role in determining the fate of an immune response. In addition, it was discovered in 2004 that neutrophils can expel their chromatin in a large, tangled web called a Neutrophil Extracellular Trap (NET). NETs are webs of chromatin crosslinked with histone and decorated with proteases, cytokines, and cathespins. These webs can trap and sequester bacteria so it is thought that bacteria trapping is a major function of NETs. However, extracellular histones, DNA and proteases can interfere with autoimmune function, damage native tissue, and stimulate aberrant inflammatory responses, meaning that NETs are implicated in a variety of inflammatory conditions including sepsis, ARDS, cystic fibrosis, heart disease, and systemic lupus erythematosus.
Because neutrophil NET composition is determined by the cell, it is difficult to study how individual components that make up NETs influence the effect of NETs in health and in pathophysiology. To address this, our lab developed a NET-mimicking DNA-based biomaterial with tunable composition that can act as a minimal-component NET for studying how the addition of different NET components influences functions such as pathogen trapping and immune cell priming. So far, we have shown that our “microwebs” composed of salmon DNA and calf histone can trap bacteria and activate dendritic cells. Going forward, we are continuing to use our platform to investigate NETs in a variety of diseases.
Lab members: Midori Maeda, Katherine Nguyen
Collaborators: Hong Sun Kim, Jason S. Knight, James Moon
References: Song 2019, Weerappuli 2019
Microphysiological Systems
Combining physical forces, engineered materials, and multiple cell types to recreate the tissue microenvironment from the bottom up.
Bioassays
Invention and improvement of protein- and cell-based assays that quantify cellular responses to drugs and organotypic stimuli.
Bioprocessing and micro/nanofabrication
Separations and spatial manipulation of biological material such as cells, proteins, nucleic acids, and bacteria.